Once a symbol of China’s culture, bikes use slumped with the country’s economic and urban development. The country’s streets have seen a mass influx of cars and China became the largest automobile market in the world. Commuting by bikes started to be perceived as a symbol of belonging to a lower class, while owning a car represented a higher economic and social status of an individual. However, this trend seems to be changing again, along with commuters’ view on using bike as a mode of urban transportation. With sharing economy on the rise, mobile app-based bike sharing starts to appeal especially to younger Chinese, who perceive biking across the country’s cities cool. Given the congested roads and poor air quality, the government is ready to embrace such greener bike renting services that offer eco-friendly alternative to cars and have the potential to reverse the way people commute thereby reshape the dynamics of the cities in China.
Previously known as the ‘kingdom of bicycles’, bikes were China’s major mode of transportation from 1980 to 2000. However, over time, the economic boom led to a high demand for cars making two wheelers go out of fashion. In 1980, around 63% of people in China used bicycles for their commute. By 2000, the rate dropped to 38% and as of 2016, it was below 12%. In 1995, there were estimated 670 million bikes in China, a figure which fell to 370 million in 2013. But now, the new bike sharing apps are likely to help promote the reversal of this trend in China, a country which has been making efforts to promote cars in the last two decades.
While the country already witnessed the emergence of innovative bike sharing apps over the last few years, bicycle sharing is still set to become a vital focus area for several China’s start-ups in 2017. This new bike-sharing service borrows from the known public bike concept present both in China and in several cities across the world, but unlike the government-run bike rental programs (also present in various cities in China), these bike sharing start-ups offer bikes equipped with GPS. GPS lets the user know which bike is in the user’s vicinity and allows to hold the bike for up to 15 minutes until the user arrives at the location where the bike is parked. The bike is unlocked by scanning a QR code and the GPS device is charged by pedaling the bike.
Popularity of such apps is fueled by the rising demand for means to commute on short distance, currently an underserved area of public transportation. For instance, in Beijing and Shanghai, white-collar workers depend primarily on public transportation for their daily commute, which leads to an increased demand for means to commute from their home or their workplace to the nearest subway or bus station. Bike rental caters to this demand without any complicated procedure or heavy deposit required (since these apps offer bikes with minimal and refundable deposit). In addition, the bike rental concept is becoming a hit with college and school students who cannot afford or try to avoid the responsibility of owning an asset, and also consider biking around the city cool.
Furthermore, the bike rental industry resonates with the growing awareness of an urgent need to address the issue of dramatically deteriorating air quality in most Chinese cities by offering eco-friendly substitute to cars, thereby fighting China’s increasing traffic and air pollution problem.
As of January 2017, there were around 17 companies operating in this new bike rental sector of sharing economy market, out of which MoBike and Ofo are the leading players attracting investment from foreign companies. In January 2017, MoBike raised US$ 215 million from a range of investors including Warburg Pincus, Tencent Holdings, and Ctrip.com International. In February 2017, the company raised additional funding from Singapore-based investment company Temasek Holdings and investment group Hillhouse Capital. The start-up also announced it raised an undisclosed amount from Taiwan-based electronics contract manufacturing company, Foxconn, in January 2017 with a view to increase its fleet size to reach 10 million new bikes every year. The association also aims to cut down the overall production cost and reduce the distribution cost of placing bikes internationally by setting up production houses in strategic locations.
MoBike’s competitor, Ofo, has also been in the limelight for attracting investments from tech players. China’s leading ride-hailing start-up, Didi Chuxing, investment group DST Global, and a private equity firm CITIC are some of the investors in Ofo, which has raised a total of US$ 450 million as of 2017.
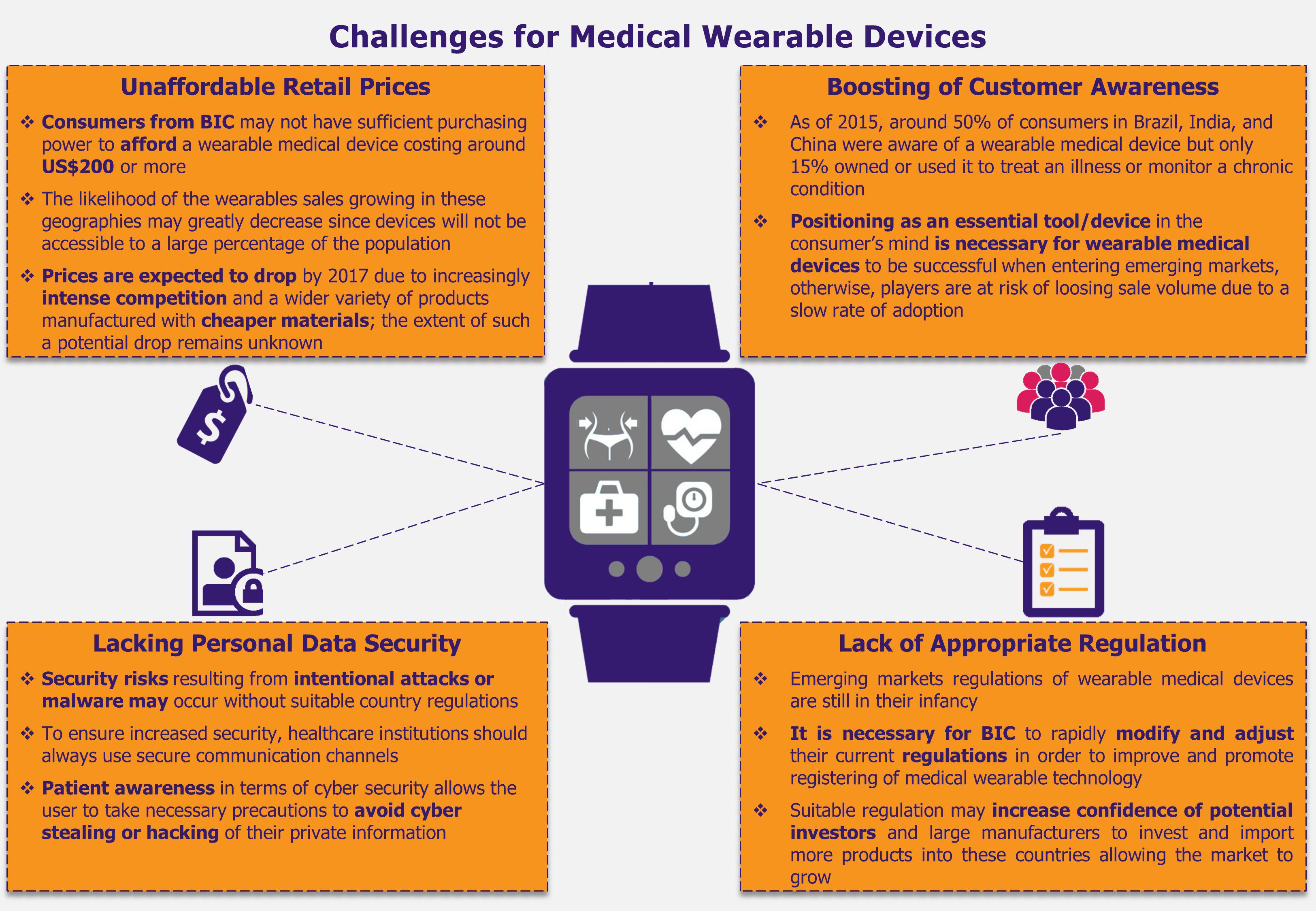
App-based bike rental industry has become one of the hottest sectors in China, leading to various domestic and international investors eager to cash in, funding new start-ups operating in this market. The start-ups compete majorly on price and the range of services offered with regards to finding and unlocking bikes, and aim to increase their presence throughout China and abroad. Despite the increasing investment, these start-ups are facing challenges which might hinder the growth of the industry. At present, no industry-specific regulations have been laid out for the bike renting services, including framework of rules and criteria qualifying companies as bike-renting operators, monitoring their activities, or setting up bike maintenance requirements, which can in turn affect user safety. In addition, start-ups face the constant fear of theft and vandalism of their fleet, which can lead to considerable expenses. For instance, in March 2017, around 4,000 bikes were found to be illegally parked in public areas in Shanghai and were confiscated by local authorities. Most of the bikes were owned by MoBike which is now required to pay a management fee and hopes to get 3,500 bikes returned.
Further, the ‘park anywhere’ policy followed by most operators is a double-edged sword, mostly due to users’ negligence with regards parking the bikes. While the policy increases the chances of bikes being found in immediate vicinity, a fact appreciated by the users, it also agrees to bikes being parked in remote areas. As a result, cities in China witness bikes being piled up along freeways and private buildings, and also obstructing pedestrians on the sidewalk.
EOS Perspective
Internet-based bike renting business is booming at an unprecedented rate and adding new momentum to the push to build up the country’s green transportation system. Investors are ogling opportunities presented by the growing platform which leverages on millions of young and tech savvy users. The bike rental start-up battle has just started and the competition is helpful in boosting the size of the industry. Start-ups such as MoBike and Ofo are thriving in the short run by offering new services which let the commuter rent a bike with the help of a mobile phone. However, the increasing investment is not enough for long-term viability and external support in the form of regulations and parking rules is required.
In order to grow, bike rental operators have to resolve issues such as bike theft, vandalism, and disorderly parking. For instance, to avoid bike theft, MoBike has hired staff patrols to keep a check on the bikes. However, this generates additional costs and is only partially able to address the issue of bike theft, as these patrols are unable to monitor all bikes at any point of time. In addition, MoBike introduced a credit score system for users to avoid damage to the bikes, by increasing the user’s responsibility for equipment. Under this system, penalty points are taken in case of any vandalism. Once a user’s score falls below a certain level, the rental fee is increased.
Bike rental companies also need to work on their business model for long-term viability. Despite booming in the short run by offering new and innovative services, these companies will need to overcome a major challenge around the profitability model – tackling it either through in-app advertising, government subsidies, or expanding to other ancillary services. It seems that long-term growth based exclusively on rental fees might be limited as soon as streets in key cities become saturated with this kind of service. Further, the industry is yet to solve problems such as the limited number of bikes a city can handle, as the road space and bike-dedicated lanes might not expand fast enough in most Chinese cities. Unless these companies come up with a long-term plan for sustainability, the future of the industry is hard to predict.