It took 60 days for global COVID-19 infections to reach 100,000, but this figure doubled in the following 12-14 days, and the addition of next 100,000 cases took only 3 more days. Because of highly contagious nature of the novel coronavirus, testing became essential to keep the epidemic under control. As a result, there was a spike in global demand for coronavirus testing kits. As per McKinsey’s estimates, in May 2020, global demand for coronavirus testing was 14 million to 16 million per week, but less than 10 million tests were being conducted.
Industry was quick to respond to the rise in demand
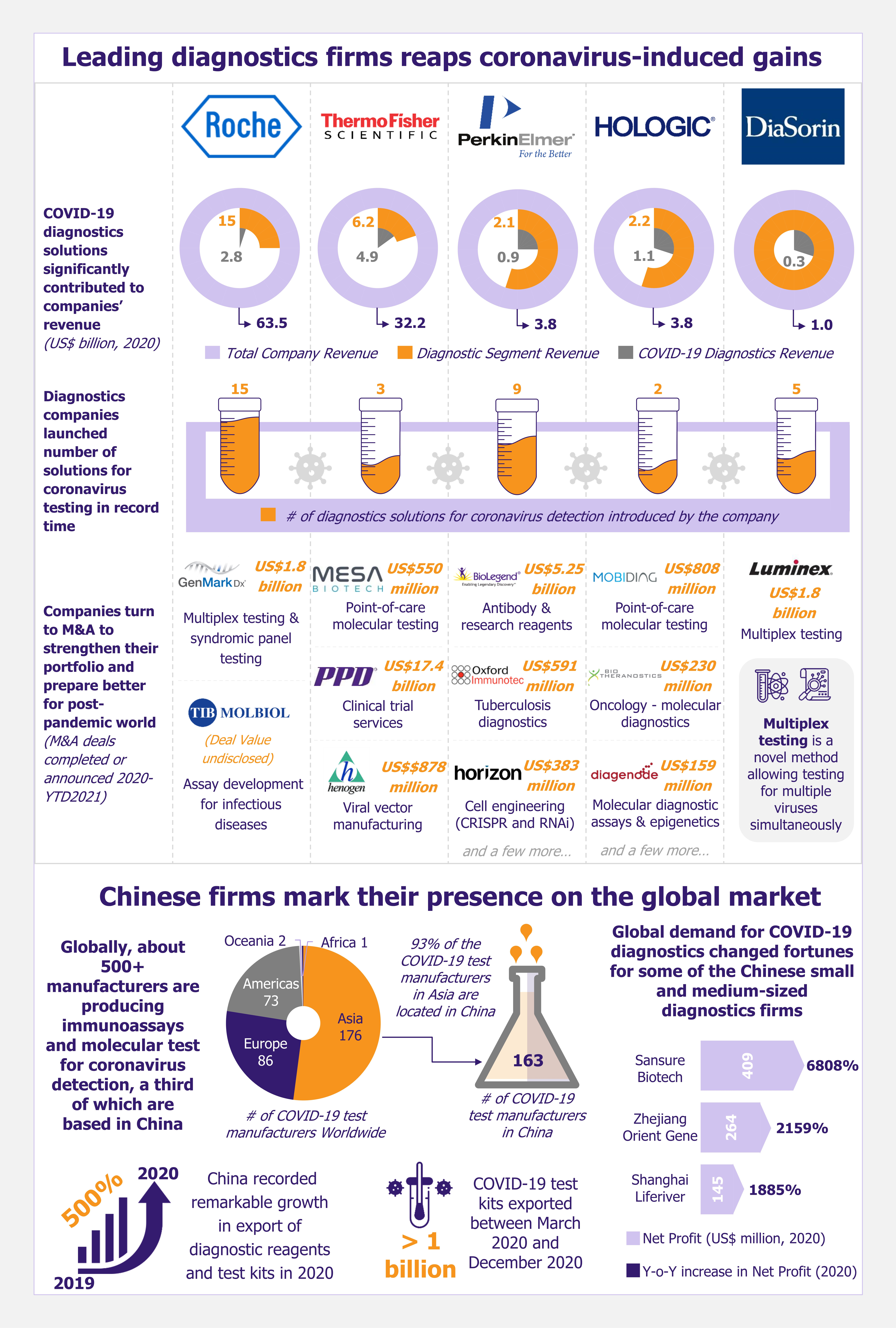
The widespread outbreak of coronavirus required the manufacturers to develop and launch new testing kits in large volumes in a short duration of time. Diagnostics kit suppliers responded promptly to this spike in demand by developing new coronavirus testing kits. Roche Diagnostics, for instance, developed coronavirus test in about six weeks – such diagnostic tests generally take 18 months or more to reach regulatory review stage. In 2020, Roche developed a total of 15 solutions for coronavirus diagnosis.
Governments across the world eased up regulatory procedures for manufacturers in order to allow rapid development and commercialization of the coronavirus testing kits. This paved way for many companies to quickly launch new products to the market. For instance, a Korean firm, Seegene, developed coronavirus testing kit in two weeks and got approval from Korea Centers for Disease Control and Prevention (KCDC) in another two weeks’ time. Such approvals generally take more than six months in Korea.
Furthermore, standard regulatory process for approval of diagnostic kits in the USA typically take several months, but considering the public health emergency in the event of pandemic, the FDA issued emergency use authorizations to expedite the process of bringing coronavirus test kits to the market. Emergency use authorizations are like interim approvals provided on the basis of sufficient evidence to suggest a diagnostics test is effective and the benefits outweighs potential risks.
By the end of 2020, the FDA granted emergency use authorization to 225 diagnostic tests for coronavirus detection, including test kits developed by Abbott Laboratories, Roche, Cepheid, Clinomics, Princeton BioMeditech, UPenn, Inno Diagnostics, Ipsum Diagnostics, Co-Diagnostics, QIAGEN, DiaSorin, BioMérieux, and Humanigen.
Leading companies with adequate resources quickly ramped up their production capacity by multifold in line with the rising demand. For instance, a US-based firm, Thermo Fisher Scientific, increased the global production of coronavirus test kits from 50,000 per week in January 2020 to 10 million per week by June 2020. In 2020, Roche spent CHF 137 million (~US$149 million) to ramp up production capacity and supply chain for all COVID-19-related testing products.
Some companies also received government grants and private investment to scale up their production capacity. For instance, in July 2020, BD (Becton, Dickinson and Company) received a US$24 million investment from the US government to scale up production of coronavirus test kits by 50%, thereby, enabling the company to produce 12 million test kits per month by the end of February 2021.
The pandemic encouraged the shift towards decentralizing diagnostics
While the test kit manufacturers were trying to achieve round the clock production to meet the demand, they struggled with global supply chain disruptions which were also induced by the pandemic.
Coronavirus testing requires several components including specialized chemicals and laboratory testing equipment. Roche, for example, manufactures coronavirus tests in the USA but procures components of the test kit from different countries. One of the important components of test kits is reagent, a specialized liquid used for the identification of coronavirus. Roche produces these reagents mainly in Germany and few other production sites located across the world.
Further, the test kits are often compatible only with company’s own testing equipment and systems. For instance, the Roche cobas SARS-CoV-2 test kit runs on the cobas 6800 or 8800 systems. The cobas 8800 system includes approximately 23,000 components which are procured from different parts of the world. In addition to this, the production involves 101 sub-assemblies and accumulated assembly time of about 450 hours each. Final production of these instruments from Roche takes place in Switzerland.
Manufacturing of a coronavirus testing kit involves complex supply chain. Spread of coronavirus forced countries to implement extreme measures including lockdowns and trade restrictions which impacted the supply chain of test kit manufacturers. Producing all the testing components and equipment at one place is near to impossible. For instance, the production of reagents involves highly sophisticated and sensitive processes, and thus, setting up a new production site to manufacture reagents on a large scale would take several months. Setting up a new production site and streamlining the procurement for such testing equipment and systems would take several years. Hence, the diagnostics firms upped their R&D activities in an effort to develop tests that could be conducted without sophisticated laboratory systems and equipment.
Moreover, the high demand for testing compelled the diagnostics practices to evolve far beyond the traditional laboratory-based business model. The need for community testing during the pandemic that challenged the operational capabilities of hospitals and diagnostics labs dictated the importance of decentralizing diagnostics for improved patient care. This gave rise to increased demand for point-of-care testing.
The two most widely used diagnostic tests for coronavirus detection are Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Antigen tests. RT-PCR test detect viral RNA in samples from the upper and lower respiratory tract, while antigen test is used to detect viral proteins in samples.
RT-PCR test is considered gold standard for coronavirus detection since the accuracy and reliability is high compared to Antigen test. However, RT-PCR test needs to be processed in a laboratory-setting and had turnaround time of several hours. Hence, there was a need for development of RT-PCR tests that could give faster results without the support of laboratory equipment.
On March 18, 2020, Abbott announced the launch of their first coronavirus test kit that was compatible with their system ‘m2000 RealTime’ which processed 470 tests in 24 hours and another ‘Alinity m’ system with capacity to run 1,080 tests in a 24-hour period. Since there was demand for more portable and fast testing solution, on March 30, 2020, Abbott launched a RT-PCR point-of-care test that ran on ID NOW system, which is the size of a small toaster. The test delivers results in 13 minutes or less. The test price is in the range of ~US$100.
Further, despite the limitations of accuracy and reliability, in some cases antigen test is preferred because there is no requirement of a lab specialist to conduct this test, thus making it less expensive, and the result is available in a few minutes. The industry saw an opportunity here and quickly developed rapid antigen tests that can be conducted at home without any assistance. For instance, in December 2020, the US FDA granted emergency use authorization to an Australia-based firm Ellume’s antigen test (priced at ~US$30) as first over-the-counter at-home diagnostic test for coronavirus detection. Soon after, Abbott also received emergency use authorization from FDA for its at-home rapid antigen test (priced at US$25) giving results in 15 minutes.
Other countries around the world also followed the suit by extending official authorization to the home-based tests for coronavirus detection. For instance, in February 2021, Germany’s Federal Institute for Drugs and Medical Devices (BfArM) granted special approval for the first time to antigen home-test kits developed by US-based Healgen Scientific as well as China-based firms Xiamen Boson Biotech and Hangzhou Laihe Biotech.
Coronavirus crisis accelerated innovation in the field of diagnostics
In a united fight against the pandemic, governments, private sector, as well as NGOs and philanthropists across the world stepped forward to raise funds to bolster R&D efforts in coronavirus diagnostics. As per data compiled by Policy Cures Research (an Australian firm engaged in global health R&D data collection and analysis), from January 2020 to September 2020, funds worth over US$800 million were committed for coronavirus diagnostics R&D. The firm also indicated that 450+ coronavirus diagnostics products were in R&D pipeline since January 2020 to December 2020.
With firms looking to capitalize on exponentially rising demand for coronavirus testing, the development of new diagnostics technologies beyond conventionally used tests (i.e., RT-PCR and antigen tests) picked up significantly.
For instance, in May 2020, the FDA granted an emergency use authorization to first ever CRISPR-based rapid test kit developed by Sherlock Biosciences. CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, is a gene editing technology which allows to alter the DNA. Sherlock’s rapid test is a paper-strip test (like a pregnancy test) which can be conducted at point-of-care and does not require any additional equipment for processing of the test. The test works by programming a CRISPR enzyme to release a detectable signal in presence of genetic signature for coronavirus.
In March 2020, US-based Surgisphere launched a smartphone app using Artificial Intelligence algorithms to detect coronavirus infection. This app confirms diagnosis by integrating the findings of chest CT scan and laboratory tests with clinical symptoms and exposure history. Preliminary studies found that the tool can detect coronavirus infection with 95.5% accuracy.
Further, application of nanotechnology for diagnosis of coronavirus infection is also underway. Canada-based Sona Nanotech developed a rapid antigen test using gold nanoparticles. This is a strip test that can be conducted at point-of-care and gives result in 15 minutes. Research is in progress to develop wearable sensors using nanoparticles for detection of coronavirus. In January 2021, University of California San Diego received US$1.3 million from the National Institutes of Health to develop a test strip containing nanoparticle that change color in presence of coronavirus. This test strip can be attached on a mask and used to detect coronavirus in a user’s breath or saliva.
Innovation wave was not limited to development of different types of tests but also expanded to consumables. For instance, in March 2020, HP (a company manufacturing 3D printers) teamed up with Beth Israel Deaconess Medical Center (a teaching hospital of Harvard Medical School) to develop 3D printed nasopharyngeal swab (typically used to collect sample for coronavirus testing) and within 35 days the clinically validated swab was ready for use. By May 2020, these swabs were commercially available for the US market following the FDA approval. In June 2020, a Belgium-based 3D printing service provider, ZiggZagg, began to plan large-scale production of swabs on their fleet of HP 3D printers. By October 2020, the company had 3D-printed over 700,000 swabs for the Belgian market.
EOS Perspective
A market research firm, The Business Research Company, estimated that the global COVID-19 rapid test kits market was expected to reach a value of US$14.94 billion in 2020. Due to worldwide vaccination drive, the market is expected to decline at a rate of -54.9%, to reach US$1.37 billion in 2023.
Though the demand for coronavirus tests is expected to diminish eventually, it has supported rapid development of diagnostics infrastructure which will remain. In India, for example, only one laboratory was performing molecular assays for COVID-19 in January 2020. The COVID-19 pandemic has shifted that balance. By May 2020, some 600 Indian RT-PCR laboratories had been set up in an effort to help manage the pandemic, thousand-fold increasing testing capacity. The additional capacity will likely remain in place as the pandemic subsides, leaving the RT-PCR assay as the dominant method for diagnosing most viral infections in India in the future.
Furthermore, with surge in demand for the coronavirus testing, the provision of diagnostic services expanded beyond the purview of hospitals and laboratories. Mobile testing facilities and drive-through testing sites propped up with development of point-of-care diagnostics. For instance, Walgreens, one of the largest pharmacy chains in the USA, offer coronavirus drive-thru testing at 6,000+ locations across the country. Further, there is high-demand for home-based testing.
Diagnostics firms riding high on the COVID-19 gains have been actively scouting opportunities to strengthen their positioning in the market and prepare for the post-pandemic world. High demand for COVID-19 test kits boosted the revenues of diagnostic companies, with Roche, Thermo Fisher, PerkinElmer, Hologic, and DiaSorin among the companies benefiting. With strong balance sheet, these companies went on with M&A flurry to advance their diagnostic portfolio and other core business verticals.
As the virus originated in China, the country was better prepared and first to develop relevant detection mechanisms. By the time the virus spread to the other parts of the world, Chinese companies were ready to export detection kits globally. Coronavirus outbreak helped China to penetrate major markets such as EU and the USA in which the indigenous diagnostics companies traditionally had a stronger hold. China was a net importer of diagnostic reagents and test kits in 2019. But in 2020, after the outbreak of coronavirus, China ramped up its production capacity of diagnostic reagents and test kits, and as a result its export growth increased by more than 500% and the country became a net exporter of diagnostic reagents and test kits by the end of 2020.
This indicates that the outbreak of the pandemic has shifted the market dynamics on many fronts. As the pandemic slowly subsides, some of these shifts might partially revert, however, the way testing is performed is likely to remain.