The revision of the EU pharmaceutical legislation represents a major achievement for the pharmaceutical sector within the European Health Union. The European Health Union, established in 2020 as a collaboration among EU member states, aims to effectively respond to health crises and improve healthcare systems across Europe. This revision provides an opportunity for the pharmaceutical sector to adapt to the demands of the 21st century, enabling greater flexibility and agility within the industry. The updated EU pharmaceutical legislation places a strong emphasis on patient-centered care, fostering innovation, and enhancing the competitiveness of the industry.
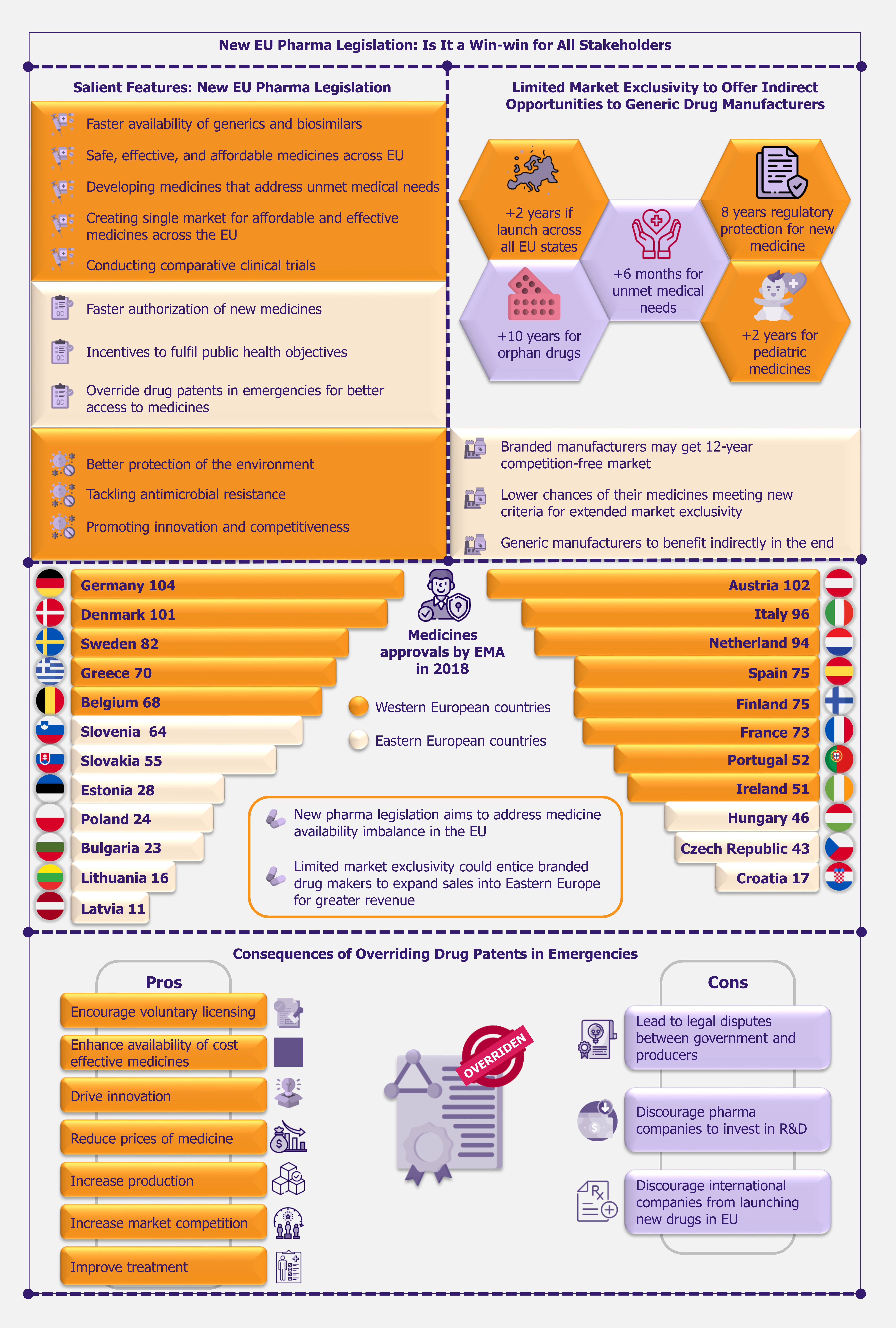
Limited market exclusivity to offer indirect opportunities to generic drug manufacturers
The COVID-19 crisis in 2020 raised a significant concern related to the accessibility and availability of life-saving medicines. The pandemic highlighted the significance of establishing effective incentives for the production of medicines to address medical needs during health emergencies.
Therefore, revised EU pharmaceutical legislation includes several rules and regulations to incentivize pharmaceutical companies to create a single market for medicines to ensure equal access to affordable and effective medicines across the EU. This is to be achieved through reducing the administrative burden by shortening authorization time, the duration required to review and grant approval for a new medicine, ensuring efficacy, safety, quality, and regulatory requirements. For example, the EU Commission will have 46 days instead of 67 days for authorization of medicine, whereas EMA (European Medicine Agency) will have 180 days instead of 240 days for the assessment of new medicine.
The new directive incentives are expected to help in improving access to medicines in all member states, in developing medicines for unmet medical needs, and in conducting comparative clinical trials (CCT). Comparative Clinical Trials are clinical research studies aimed at comparing the efficacy and safety of distinct medical treatments. Such trials usually entail two or more groups of participants, each receiving a different treatment in order to ascertain the more effective, safer treatment that offers better outcomes for a specific condition.
The legislation also focuses on maintaining the availability of generic drugs and biosimilars to help countries with more affordable and accessible medicines across the EU. It also aims to provide enhanced rules for the protection of the environment, such as mandatory ERA (environmental risk assessment) of medicines which focuses on discarding medicines properly by ensuring the minimization of environmental risks that are associated with the manufacturing, use, and disposal of medicine on the EU market, promoting innovation, and tackling antimicrobial resistance (AMR).
The revised pharmaceutical legislation introduces a shortened period of regulatory protection, reducing it from 10 to 8 years, in order to establish a unified market for new medicines. This protection encompasses 6 years of regulatory data protection and 2 years of market protection. Companies can also benefit from an additional 2 years of data protection if they launch their medicine in all 27 EU member states and an extra 6 months of protection if their medicine addresses unmet medical needs or undergoes comparative clinical trials.
The revised EU pharma legislation also includes provisions for 2 years of market exclusivity for pediatric medicines and 10 years of market exclusivity for orphan drugs. The limited market exclusivity for branded drug manufacturers is expected to give the generic medicine makers more opportunities for production, hence improving the affordability and accessibility of medicines across the EU.
Assessing changes for the European Medicines Agency
The EMA is responsible for the evaluation and approval of new medicines while monitoring the safety and efficacy of the medicine. The revised EU pharmaceutical legislation has bestowed significant responsibilities upon the EMA. These responsibilities encompass expediting data assessments and providing enhanced scientific advice to pharmaceutical companies. The legislation has both positive and negative impact on the EMA.
On the positive side, it aims to harmonize regulatory processes across member states, leading to a more streamlined and efficient system. This is expected to improve the agency’s ability to assess medicines promptly, facilitating faster access to innovative treatments. Additionally, the legislation encourages collaboration among regulatory authorities and promotes international partnerships, which strengthen the EMA’s regulatory capacity and scientific expertise. Further, the new regime is likely to foster EMA to prepare a list of critical medicines and ensure their availability during shortages.
The challenges that EMA might face if the new pharma legislation is passed include increased workload and resource requirements, which may necessitate additional staff, expertise, and funding. Complex areas such as pricing, pharmacovigilance, data transparency, and reimbursement could pose difficulties, potentially leading to delays and discrepancies.
Balancing affordability and access to medicines while incentivizing pharmaceutical companies’ investment in R&D under strict regulations, health technology assessments, and data transparency could be a challenge. EMA might face obstacles in training, resource allocation, and maintaining regulatory consistency. Both positive and negative impact should be considered while implementing the revised legislation.
Overriding drug patents could ensure supply, albeit with challenges
Overriding a drug patent is a legal mechanism allowing governments to bypass the patent protection of medicines and medical technology during emergency situations.
Although it poses challenges to the original patent holder company, including implications on revenue streams, investments, and profitability, it enables the granting of compulsory licenses to generic drug manufacturers, which increases production and reduces prices, particularly during health emergencies, while still considering the rights and interest of patent holders (through compensation for the use of their invention during the emergency period). It also encourages voluntary licensing that allows generic manufacturers to produce and sell products with the patent holder’s permission while respecting patent rights, instead of overriding the patent as it is in compulsory licensing.
Amidst concerns pertaining to intellectual property (IP) rights and the fact that this move might potentially discourage pharma companies from investing in R&D initiatives, the revised EU pharmaceutical legislation proposes overriding drug patents, as it would enhance the availability of affordable and cost-effective medicines throughout the EU. The production of generic drugs and biosimilars is likely to help increase market competition, drive innovation, and introduce improved treatments across the EU, maintaining a competitive edge.
Overriding drug patents might also have ramifications on international trade and relationships, leading to disputes and strained ties between countries. While considering these laws, policymakers need to exercise caution to ensure both accessibility of medicines and adequate investments in R&D.
New EU pharma legislation to benefit Eastern European countries
The difference in access to medicines between Eastern and Western European countries is evident from the fact that from 2015 to 2017, EMA approved 104, 102, and 101 medicines for Germany, Austria, and Denmark, respectively, compared to only 24 in Poland, 16 in Lithuania, and 11 in Latvia. These distinct differences in the availability of medicines between Eastern and Western European countries could be attributed to factors such as stronger healthcare systems in the Western region, higher healthcare budgets, and a greater ability to negotiate pricing and reimbursement agreements with pharmaceutical companies.
Western European countries have relatively better funded and more advanced healthcare infrastructure, including clinics, hospitals, and specialized healthcare services compared to Eastern European countries. Western European countries have a larger capacity to invest in research and development and contribute to the development of new medicines.
Moreover, differences in national healthcare policies contribute to the variation in pharmaceutical benefits and outcomes. The presence of a robust and extensive pharmaceutical manufacturing industry in Western European countries allows for faster production and distribution of medical supplies. Consequently, Western European countries generally have better access to medicines and medical supplies compared to Eastern European countries.
The new EU pharmaceutical legislation helps Eastern European countries by reducing the exclusivity period of newly introduced drugs. This measure can prevent branded drug manufacturers from selling drugs exclusively to more affluent countries.
Moreover, according to experts, branded drug manufacturers are likely to only theoretically benefit from a competition-free market for 12 years because the majority of medicines launched by them are unlikely to meet all the new criteria in order to be granted this extended competition-free market access. This might compel branded medicine manufacturers to expand their sales base and sell in Eastern European countries as well to maximize their revenues.
New EU pharma legislation to spur a changing investment landscape
With the approval of new EU pharmaceutical legislation, it is expected that investment plans within the pharmaceutical sector will undergo significant changes. The regulatory changes, which aim to reduce the time and administration burden, could help in attracting lucrative investments by offering faster returns for pharmaceutical companies.
The new legislation can be expected to bring more investments in the R&D and manufacturing sectors by addressing critical healthcare challenges. Furthermore, the availability of generic and biosimilars would also help by creating opportunities for investment in the production/manufacturing of cost-effective medicines.
Moreover, enhancement in transparency and data sharing can also lead to increased collaboration and partnerships in R&D, attracting investments from the public and private sectors in the medical space.
However, investment plans could vary depending upon various factors such as intellectual property rights, market dynamics, competitive landscape, etc. Pharmaceutical companies need to assess new legislation in order to adjust their investment strategies to navigate potential challenges.
EOS Perspective
Analyzing the winning stakeholders of the revised EU pharma legislation could be challenging at this point in time owing to the fact that the new regime focuses on addressing issues of affordability and innovation across the EU which tend to be contradicting. These aims are to be achieved by incentivizing R&D and manufacturing sectors, enhancing market competition, and promoting collaboration.
It cannot be denied that there will be several challenges while enforcing these changes. A few of these challenges include maintaining intellectual property rights, marrying affordability with innovation, and addressing the specific needs of various patients in different countries. Specific resources and coordination will be required to overcome these hurdles. As a result, the success or failure of the EU pharmaceutical legislation for stakeholders will depend on the legislation’s actual implementation, adaptation to changing market dynamics, stakeholder engagement, as well as whether the balance between accessibility, affordability, and innovation while maintaining competitiveness is achieved and maintained in the long term.