Africa is currently facing significant challenges related to limited accessibility to vaccines as well as ongoing vaccine hesitancy. African CDC has identified these problems and is taking concrete steps to achieve its 2040 target of 60% of vaccines available on the continent to originate from domestic production. India is one of the key countries invested in the growth of Africa’s healthcare sector both financially and logistically. Due to similar geographies, climates, disease prevalence, and economies, Africa could take guidance, collaborate, or replicate Indian vaccine manufacturers’ strategies and mechanisms to scale up its vaccine sector.
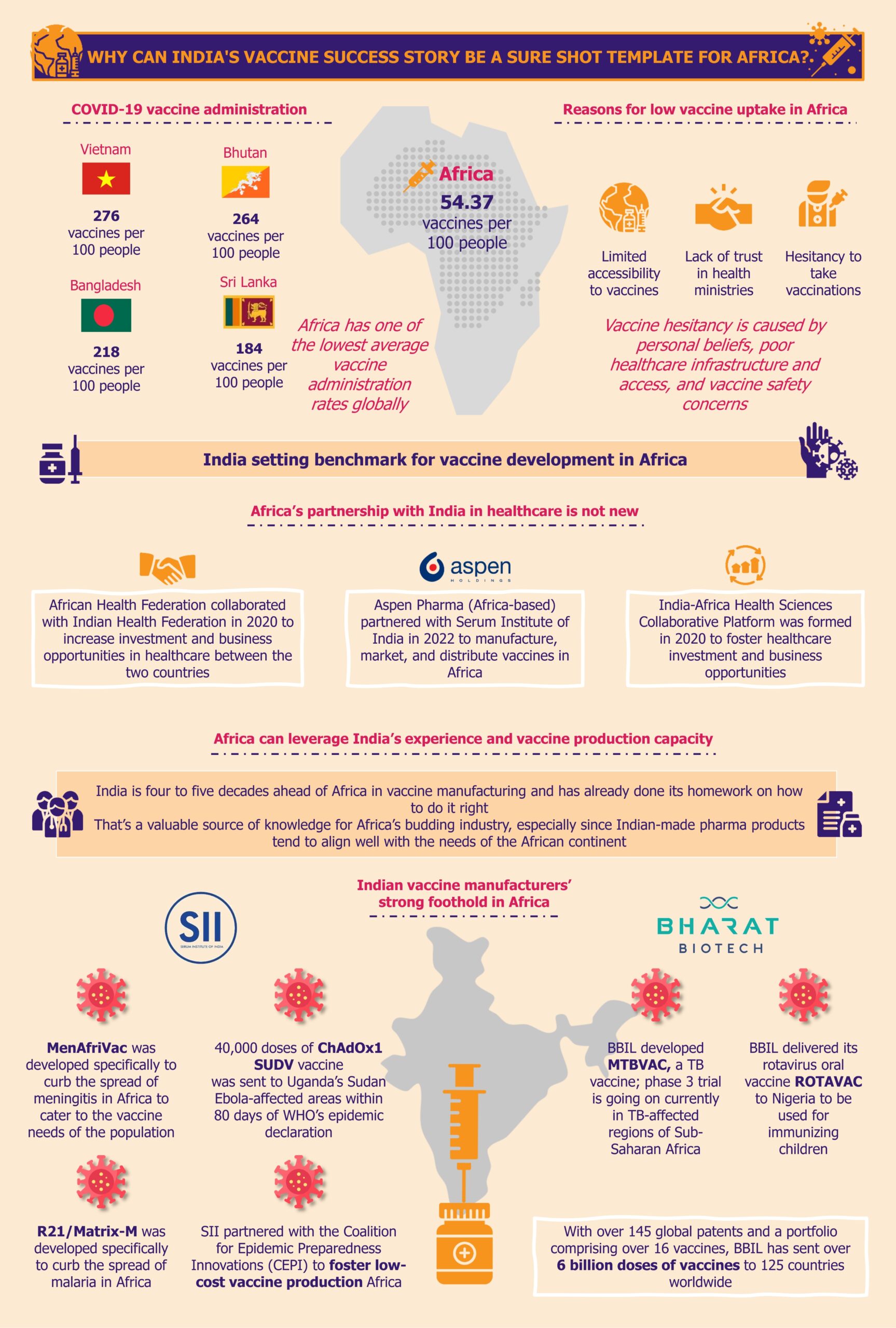
Africa has one of the lowest average vaccine administration rates globally
Unbalanced access to vaccines in Africa compared to other regions became quite vivid during the COVID-19 pandemic. Africa’s average number of coronavirus vaccine doses administered per 100 people was 54.37 as of March 15, 2023. Seychelles administered the highest number of vaccine doses at 205.37 and Burundi the lowest at 0.27.
In contrast, the world average stood at 173, with high-income countries such as the USA and Canada administering 191 and 258 vaccine doses per 100 people, respectively. Interestingly, Cuba, despite being an upper middle-income economy, administered 385, a higher number of doses per 100 people than some high-income countries.
Even some low-income economies such as Vietnam (276), Bhutan (264), Bangladesh (218), Nepal (213), and Sri Lanka (184), among others, administered a higher number of coronavirus vaccine doses than the world average (173), and far more than Africa’s average.
These stark variations in the vaccine administration rates across countries could be attributed to the lack of easy accessibility, especially in Africa, apart from other factors such as vaccine hesitancy.
Africans’ vaccine hesitancy slows down the uptake of vaccination
Vaccine hesitancy is caused by several factors such as personal beliefs, misinformation or myths, healthcare infrastructure and access, religious and cultural beliefs, and vaccine safety concerns. These are typically the main reasons for vaccine hesitancy according to an October 2023 article published by ThinkGlobalHealth, and several of these reasons are likely to apply to the African continent.
In addition to these, another critical factor that cannot be ignored is people’s lack of trust in the health ministries, a relevant aspect in some African countries such as South Africa. This was largely due to the ministries’ involvement in procurement corruption of COVID-related aid according to an article published by GlobalData in November 2023.
Africa’s low vaccine administration rate is driven by limited accessibility
One major reason for the vaccine’s low administration rate in Africa is the limited accessibility to vaccines. This has been an ongoing issue on the continent and was not just limited to pandemics such as COVID-19 and Ebola.
The African continent is overdependent on vaccine imports, with 99% of its vaccine needs being satisfied from abroad. With a total of 13 operational production facilities across the continent, the current vaccine manufacturing industry is in its infancy in Africa and produces 1% of the continent’s vaccine supplies.
African countries have recognized this issue and begun working towards its goal of meeting 60% of the continent’s vaccine needs domestically by 2040, with interim targets of 10% by 2025 and 30% by 2030.
This article is part of EOS' Perspectives series on vaccines landscape in Africa. Read our other Perspectives in the series: Vaccines in Africa: Pursuit of Reducing Over-Dependence on Imports
With some local talent available, Africa needs the right development template
While the local vaccine industry is underdeveloped, to say the least, the continent is not entirely without the talent required to produce home-grown vaccines and other pharmaceutical products such as test kits. For instance, Senegal-based Pasteur Institute developed a US$1 finger-prick at-home antigen test for COVID-19 in partnership with Mologic, a UK-based biotech company. Although the funding came partially from the UK, local talent was predominantly utilized.
To establish a sustainable vaccine sector, Africa does not need to reinvent the wheel. It could utilize lessons and success stories of other countries that have built this industry and share similarities with the African continent.
India is one such country with a vast size, diverse cultures, geography, and administrative structures under one roof, and has a tropical climate and disease profile similar to those in Africa. Additionally, India’s symbiotic relationship with the African healthcare sector would also play a significant role in empowering Africa to leverage the expertise of the Indian vaccine sector. This could be a step in the right direction for the African continent to achieve vaccine sovereignty.
Africa’s partnership with India in healthcare is not new
Africa has a long-standing healthcare partnership with India, as the latter has been the largest supplier of generic medicines to Africa. Additionally, some US$3.4 billion worth of pharma products, i.e. close to 20% of India’s total pharma exports, went to African countries as of 2018. In 2020-2021, India’s pharma exports to Africa amounted to US$4.3 billion as per the Pharmaceuticals Export Promotion Council of India (Pharmexcil).
Between 2010 and 2019, India was also the third-largest contributor to Africa’s healthcare investment landscape, after the UK and the USA. During this period, India invested around US$210 million out of a total of US$1.1 billion in global investments into Africa’s healthcare sector, accounting for a 19% share.
In the past, African pharma companies have relied on Indian organizations to pivot and streamline their business in difficult times. For instance, South Africa-based Aspen Pharmacare could not sell a single dose of its COVID-19 vector vaccine owing to multiple factors, such as the rising popularity of mRNA vaccines. Ultimately, the company partnered with the Serum Institute of India (SII) in August 2022 to produce its vaccines to minimize business loss and idle production capacity. This is just one example showcasing opportunities where African vaccine producers collaborated with Indian vaccine makers. This kind of collaboration can also become a source of guidance and knowledge on how to create own sustainable ecosystem for vaccine production.
Collaborations between Africa and India have also extended beyond adverse situations. One example of this is a partnered research to produce a DNA-based dengue vaccine. Scientists from Bangalore and Goa in India and Nairobi and Cameroon in Africa have been working together in a partnership called the India-Africa Health Sciences Collaborative Platform (IAHSP), set up in 2019. The partnership results from a collaboration between India’s ICMR (Indian Council of Medical Research) and the African Union to create this DNA-based dengue vaccine, among other research work involving antimicrobial resistance, per a January 2022 Springer Nature article.
Furthermore, in December 2020, the Indian Healthcare Federation (NATHEALTH) and the African Health Federation (AHF) partnered to foster investment in healthcare and thus promote business opportunities in healthcare between India and Africa.
India’s pharma industry has merits to learn from
The Indian vaccine production sector is rapidly gaining steam in the global market and outpacing multinational players in this industry. A few prominent Indian vaccine producers, such as SII, Bharat Biotech (BBIL), and Biological E, have captured a considerable market share globally.
Interestingly, over 60% of the global vaccine needs in terms of volume are being satisfied by only five producers globally. Three of these five producers are based in India: Pune-based SII, Hyderabad-based BBIL, and Mumbai-based Haffkine. SII tops the list of these five global producers with a 28% volume share globally, and BBIL (9%) shares the third spot with Sanofi, followed by Haffkine (7%), as of 2019.
For many years, India has been supplying cost-effective and high-quality generic medicines and vaccines, which has earned the country the title of ‘pharmacy to the world’. The title is not exaggerated, as India alone accounts for 62% of global vaccines and 20% of global generic drugs’ production by volume as of 2023. The Indian pharma sector holds the third rank by production volume and tenth by value globally.
With an 18% share of pharmaceutical exports and vast needs, Africa is the second-largest importer of pharmaceutical products from India as of 2019.
Indian vaccines’ success in Africa proves that Indian producers understand African needs
In its quest to develop its own vaccine production sector, Africa can learn a host of aspects of vaccine production from India. This includes but is not limited to the cost-effectiveness of vaccines against diseases such as COVID-19, rabies, diphtheria, pertussis, tetanus (DPT), human papillomavirus (HPV), malaria, Ebola, and meningitis. India is four to five decades ahead of Africa in vaccine manufacturing and has already done its homework on how to do it right. That’s a useful source of knowledge for Africa’s budding industry, especially since Indian-made pharma products tend to align well with the needs of the African continent.
Serum Institute of India’s (SII) foothold in Africa
SII is now the largest vaccine producer by the number of doses manufactured and sold worldwide (over 1.6 billion doses across 170 countries in 2020), including for polio, diphtheria, tetanus, pertussis, haemophilus influenzae type b (Hib), BCG, r-Hepatitis B, measles, mumps, rubella, as well as pneumococcal and COVID-19 vaccines. According to estimates, nearly 65% of children across the globe receive at least one vaccine produced by SII.
SII has a strong foothold in Africa, with several of its vaccine products being extensively used or developed specifically for the continent’s needs.
MenAfriVac, manufactured by SII, is a vaccine to prevent meningitis and was rolled out in Africa in 2010. The vaccine was developed specifically to curb the spread of meningitis in Africa to cater to the vaccine needs of its population. The price of the vaccine is less than US$0.50 per dose, with an efficacy of 52% among 12–23-month-old children and 70% among older children and adults. Thanks to the vaccine, over 152 million people were inoculated by MenAfriVac by the end of 2013, enabling the elimination of meningitis epidemics in 26 African countries.
Another example of an India-made vaccine to particularly reduce Africa’s disease burden of malaria and cater to its people’s vaccine needs is R21/Matrix-M. SII, along with Oxford University, has produced this malaria vaccine using the technology of Novavax, a US-based biotech company. The vaccine has been approved for use by some African countries’ regulatory authorities, such as Ghana, Nigeria, and Burkina Faso, as of December 2023. According to a January 2024 press release by SII, the vaccine showed efficacy of around 78% in the age group between five- and seventeen-months children in Burkina Faso, Kenya, Mali, and Tanzania over the first year. The company is planning to roll out the 25 million vaccines produced in the coming four to five months.
In December 2022, SII acted rapidly on the Sudan Ebolavirus outbreak in Uganda by sending over 40,000 doses of the investigational ChAdOx1 SUDV vaccine in a record time of 80 days after WHO declared the epidemic.
These are some examples showcasing the fact that SII, along with other Indian producers, understands Africa’s vaccine needs, which is evident from the success of these vaccines in Africa. Consequently, it makes logical and economic sense for Africa to learn from Indian vaccine manufacturers to develop low-cost, effective vaccines.
Apart from successfully selling its vaccines in Africa, SII also actively contributes to the knowledge transfer into the continent. In January 2024, SII partnered with the Coalition for Epidemic Preparedness Innovations (CEPI) to foster low-cost vaccine production in Global South countries, including Africa (also comprising Latin America and the Caribbean, Asia (excluding Israel, Japan, and South Korea), and Oceania (excluding Australia and New Zealand)) to curb the outbreak of life-threatening diseases. CEPI is a global organization formed as a result of an international collaboration between public, private, philanthropic institutions and NGOs.
CEPI has three other members apart from SII: South Africa-based Aspen Pharmacare, Senegal-based Institut Pasteur de Dakar, and Indonesia-based Bio Farma. With this partnership, CEPI intends to capitalize on SII’s expertise in making affordable, cost-effective vaccines in record time. In this pursuit, CEPI is investing US$30 million so that vaccine developers who are already partners of CEPI can expedite technology transfers to SII within days or weeks of any outbreak. This will enable SII to produce vaccines against the impending disease.
Bharat Biotech’s (BBIL) foothold in Africa
With over 145 global patents and a portfolio comprising over 16 vaccines, BBIL has sent over 6 billion doses of vaccines to 125 countries worldwide. BBIL has produced vaccines against influenza H1N1, rotavirus, Japanese encephalitis (JENVAC), rabies, chikungunya, zika virus, and cholera. The company is also the creator of the world’s first tetanus toxoid conjugated vaccine for typhoid. In addition to these, BBIL has manufactured WHO pre-qualified vaccines, such as BIOPOLIO, ROTAVAC, ROTAVAC 5D, and Typbar TCV against polio, rotavirus, and typhoid infections, respectively.
BBIL has also been offering its products to Africa. In one of the recent examples, the company delivered its rotavirus oral vaccine, ROTAVAC, to Nigeria to immunize the country’s children in August 2022. The vaccine is expected to minimize the occurrence of the disease and death due to rotavirus among Nigerian children below the age of five years by at least 40%, according to research by the Johns Hopkins Bloomberg School of Public Health.
Another example of a vaccine made by BBIL that is aligned with the needs of the African population is MTBVAC. In March 2022, the company announced its partnership with Spain-based biopharmaceutical company Biofabri to develop, produce, and distribute MTBVAC, a novel TB vaccine. Phase 3 trial is currently underway in TB-affected regions of Sub-Saharan Africa such as South Africa, Madagascar, and Senegal. With 25% each, Sub-Saharan Africa and India account for the highest TB burden across the globe. The vaccine is being developed to target TB in these susceptible regions to eradicate the disease.
Several other Indian manufacturers have rolled out successful vaccines against various diseases in Africa that have significantly reduced the disease burden in the region.
EOS Perspective
Achieving 60% local vaccine production within 15 years will be possible only if Africa chooses a robust role model to learn from. India stands out as possibly the only near-perfect choice for that. To foster the development of a seamless and sustainable vaccine ecosystem, Africa should replicate, take guidance, and collaborate with Indian manufacturers as much as possible.
The world has evolved and many steps taken by India in the past cannot be directly transplanted into the current African scenario. However, India’s approach to building self-reliance in pharmaceutical production can undoubtedly offer valuable lessons. Direct know-how and technology transfer, collaborations, approach to talent training, production facilities management, procurement handling, supply chain management, licensing, and IP protection are critical aspects in which Africa could utilize India’s expertise and experience in vaccine making.
By choosing India as a role model and emulating its focus on nurturing a competitive pharma manufacturing industry, Africa could take a significant step towards achieving the goal of self-sufficient vaccine production.