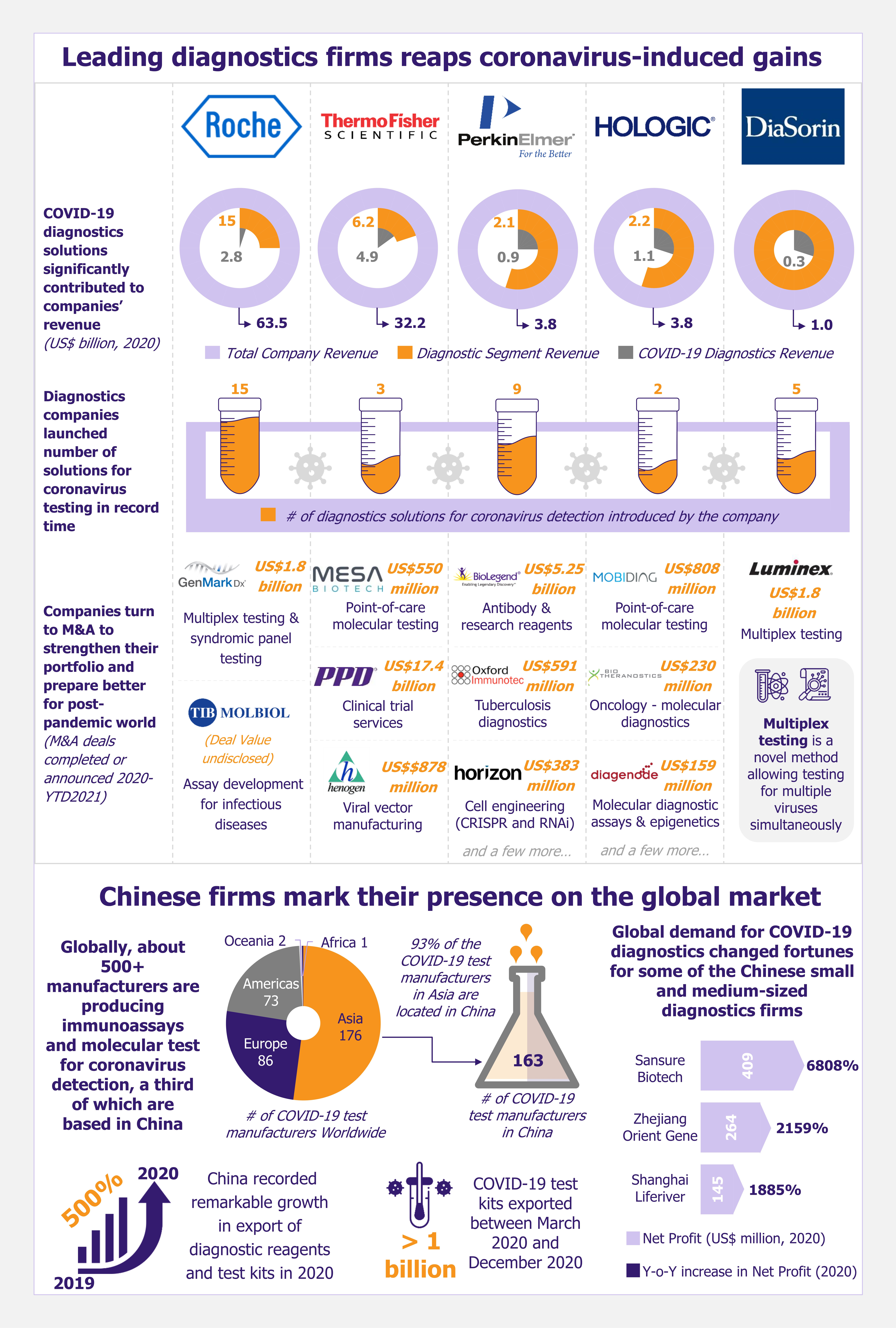
Comprehensive Genomic Profiling (CGP) is a diagnostic tool that sequences a patient’s tumor DNA to identify genetic mutations that drive cancer growth. Insurance coverage for CGP varies widely depending on the type of cancer, the patient’s stage of disease, and the specific test being used. Despite CGP’s tremendous potential to transform cancer care and diagnosis, its implementation is hindered by inconsistent insurance coverage policies.
Comprehensive genomic profiling is a cutting-edge technology that is revolutionizing cancer diagnosis and treatment. Unlike standard gene testing, which looks at a small number of genes, CGP analyzes thousands of genes across the entire genome. This provides a much more comprehensive picture of genetic mutations that may be driving a patient’s cancer, thereby leading to more personalized and effective treatment options. Despite the benefits of CGP, access to this technology remains limited due to a variety of factors, which include high costs, limited insurance coverage, and regulatory hurdles.
One of the biggest challenges for CGP has been payer acceptability. Payers tend to be cautious about covering CGP because it is a relatively new technology, and there is still some debate about its clinical value and cost-effectiveness.
Private payers in the USA are more likely to cover CGP for patients with rare or complex cancers or for patients who have failed standard therapies, such as chemotherapy or radiation therapy.
In contrast, public payers, such as Medicare, may have more restrictive criteria for coverage and only cover CGP for certain types of cancer or for patients who meet specific clinical criteria. These criteria could include a requirement that CGP tests be performed in Medicare-accredited labs. Other major public payers in the USA, such as Medicaid and Veterans Affairs (VA) health plans, also cover CGP, but each payer has different criteria for coverage. Generally, they require that the test is ordered by a physician and is deemed medically necessary for the patient’s treatment plan.
The lack of coverage makes it financially inaccessible for many patients, which limits the ability of healthcare providers to consistently offer CGP testing. This presents a significant obstacle to the widespread adoption of this promising diagnostic tool. Some payers are hesitant to reimburse CGP due to concerns about the cost-effectiveness of the test and the lack of long-term data on clinical outcomes. However, major public and private payers such as Medicare, UnitedHealth (UHC), Aetna, and Cigna, among others, have included CGP tests in their health policies in recent years, nonetheless, the coverage remains uneven.
Cost and regulatory hurdles are stifling the growth of CGP
Payers have historically covered traditional testing, such as immunohistochemistry (IHC), fluorescent in situ hybridization (FISH), and single gene tests, but have been hesitant to provide coverage for CGP. This is mainly because these tests have been around for longer than CGP, so payers are more familiar with them and are more comfortable covering them.
Another reason is that CGP is more expensive than traditional tests. While the exact cost varies depending on the specific test and lab performing it, the cost of CGP tests can range from a few hundred dollars to several thousand dollars, while traditional tests are typically in the range of a few hundred dollars. This is due to the fact that CGP tests are more complex as they analyze a large number of genes, whereas traditional tests focus on analyzing one specific gene at a time, making them less expensive.
According to a study published in 2021 by the Journal of Clinical Oncology, CGP could improve overall survival by about 6% (0.06 years, a relatively small but meaningful amount of time for cancer patients and their families) for US$9,000 per patient, compared with traditional testing strategies. On the other hand, as per a 2022 article by the American Journal of Managed Care, although the cost of CGP tests is high, these tests can help identify the most effective treatment options for each patient, which can lead to better outcomes and fewer unnecessary treatments, which in turn can lower overall healthcare costs.
Read our related Perspective: Commentary: Genetic Testing Fraud – The Next Big Concern for the US Healthcare?
To further educate the industry about the benefits associated with CGP, Illumina, a California-based biotechnology company, established Access to Comprehensive Genomic Profiling (ACGP) in 2020, which is an alliance of seven members, including leading molecular diagnostics companies, pharmaceutical manufacturers, and laboratories. ACGP aims to educate about CGP for advanced cancer patients by engaging directly with the US payers.
Additionally, a few strategies are being adopted by the healthcare industry, such as bundling CGP tests with other diagnostic tests to reduce the overall cost per test. This way, instead of running a CGP test and a separate test for a specific genetic mutation, both tests could be combined into one-panel tests. This could reduce the overall cost per test by eliminating the need to run two separate tests, as well as reducing the need for multiple lab visits and samples. However, it’s important to note that the savings may vary depending on the specific tests and the laboratory.
The ambiguity surrounding reimbursement for CGP tests among the insurers also stems from the FDA’s ongoing debate over proper classification and regulatory framework for these tests. While the FDA recognizes the potential benefits of CGP, concerns linger about its quality, accuracy, and cost-effectiveness. To address these concerns, the FDA has been working with stakeholders to establish reimbursement policies that make CGP tests accessible to patients. These stakeholders range from academic institutions (such as Mayo Clinic and Memorial Sloan Kettering) to health insurance companies (such as UnitedHealthcare and Aetna) to CGP test developers (such as Guardant Health and Foundation Medicine).
Payers’ coverage for CGP is expanding but is highly uneven
Payers, such as Aetna and Cigna, have included CGP tests in their health plans but do not cover all types of cancers. While an increasing number of payers is expanding coverage for CGP, there is a lot of variation in terms of what is covered and for which types or stages of cancer.
For instance, Aetna announced in 2020 that it would cover CGP testing for certain types of breast and colorectal cancer. However, the coverage for each type of cancer gene mutation is different.
While Aetna’s policies for CGP coverage are very nuanced, Cigna’s are complicated. Cigna‘s coverage varies depending on the type of CGP test being ordered, whether the test is considered medically necessary for the patient’s condition, and the patient’s location. Sometimes, the patient needs to meet certain criteria to be eligible for coverage (e.g., only the advanced stage of cancer is considered under coverage).
Similarly, UHC, one of the leading private health plan providers in the USA, also limited its CGP coverage to patients with advanced cancers, such as lung, breast, or colorectal cancer. However, in early 2023, UHC issued a new policy expanding coverage for CGP tests from Foundation Medicine and Guardant Health. The new policy covers CGP tests for a wider range of cancers, including early-stage cancers and other types of tumors. The goal of this policy change is to increase access to CGP testing and to help catch cancer earlier when it is more treatable.
Aetna and Cigna are not far behind in expanding their coverage for CGP tests. In 2023, both companies included additional benefits for members receiving CGP testing, such as on-site care in some facilities and counseling services.
There’s an increasing recognition that CGP can help identify patients who may benefit from targeted therapies. Overall, payers are becoming more open to covering CGP, but there is still variability in their policies and coverage levels.
EOS Perspective
The adoption of CGP is creating a ripple effect throughout the healthcare industry. Payers are increasingly recognizing the value of CGP tests and expanding their coverage. By providing broader coverage for CGP tests, payers can position themselves as offering more cutting-edge care options. This can give them a competitive edge over other insurers who may not provide coverage for these tests. In addition, by broadening the coverage of tests for early-stage cancer, payers can help to identify and treat cancers earlier, which can lead to better outcomes for patients and potentially lower costs in the long run.
Further, the growing adoption of CGP has an impact on healthcare industry stakeholders beyond payers. It is likely to fuel a shift towards precision medicine, where treatments are tailored to the individual patient based on genetic information.
Diagnostic companies are likely to invest in CGP technology to stay competitive and offer more comprehensive tests. For healthcare providers, offering CGP tests allows them to differentiate themselves, improve patient outcomes, and attract more patients. However, it can also add complexity to the treatment process and increase costs if not managed correctly (e.g., wrong interpretation of genetic information due to the large amount of data for individual patients).
For test kit producers and labs, CGP is creating new opportunities for growth and market share but also increased competition and pressure to lower costs and improve accuracy.
Overall, while still not fully embraced by the industry, CGP is shaking up the healthcare landscape, creating both great opportunities and new challenges for all stakeholders.