China’s phased rollout of Volume-Based Procurement (VBP), which started in the pharmaceutical industry in 2018, quickly expanded to the medical devices market in 2019. VBP standardizes medical device prices in China, fosters medtech innovation, makes medical consumables more accessible, and reduces healthcare expenses for both patients and the government. The program is doing this by granting high-volume sales to medical device manufacturers, offering the lowest prices across the city, province, or country level, depending on the tender type.
VBP policy follows the Healthy China strategy
In September 2021, the National Healthcare Security Administration, a governmental body responsible for funding public healthcare in China, made its 14th five-year plan (FYP), including the years from 2021 to 2025. China’s 14th FYP has laid down a Healthy China strategy that aims to improve social conditions, including healthcare in the country.
China has struggled with costly and uneven healthcare access between urban and rural areas, contributing to poverty, primarily in rural regions. Poverty due to illness affected around 20 million people, or 44.1% of the poor population in 2015, according to the National Health Commission. Other challenges include a hike in noninfectious diseases due to poor lifestyle habits and an increasingly aging population.
The 14th FYP aims to mitigate these challenges by improving the affordability and accessibility of healthcare services in China by 2025. China will achieve this through various policies, including public hospital reform and equal access to essential public healthcare services.
The Chinese government rolled out VBP, or competitive tendering of medical consumables, to achieve the 2025 target of 80% of hospitals’ expenditure to go through the provincial tendering process across several device categories.
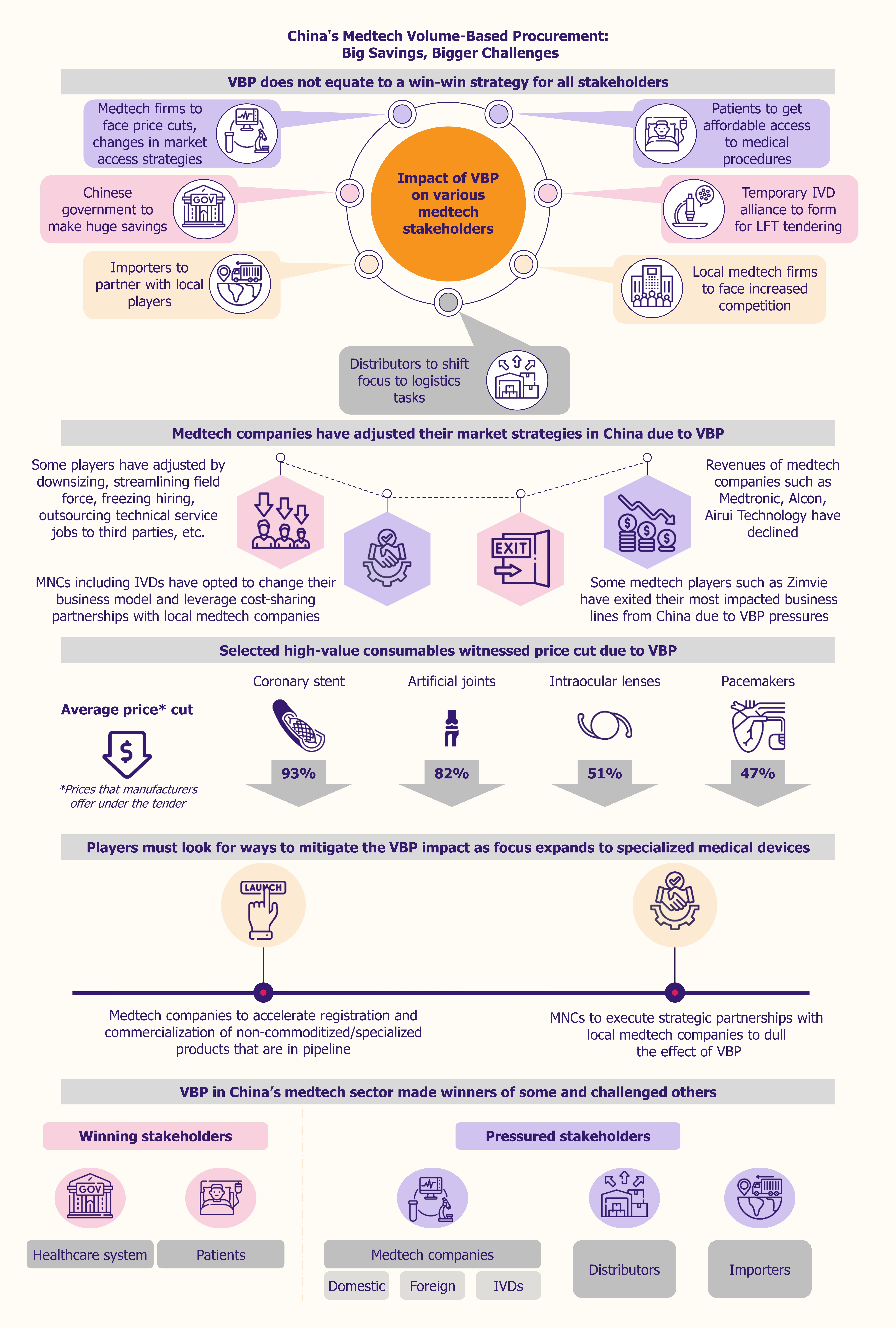
VBP has had a tremendous impact on many medtech players in China
Between 2019 and 2021, the top 10 producers of high-value medical consumables in the Chinese market participated in VBP tenders at the provincial level with a nearly 70% median reduction in the prices the manufacturers offer under the tender.
The average price reductions on medical devices such as coronary stents, joint replacement systems, spinal and orthopedic products, and total knee replacement systems were cut by 95%, 82%, 84%, and 84%, respectively.
With these significant price reductions, VBP rippled through the medtech sector, affecting companies in many ways and forcing them to rethink their strategies to compete more effectively in China.
Declining revenues
The introduction of VBP has adversely affected some of the leading medtech companies, resulting in a downturn in their revenues in China. These medtech companies aimed to mitigate the impact by reducing costs and discounts. The impact of VBP on the world’s largest medical device maker, Medtronic, caught the attention of the medtech community due to the company’s considerable presence in China. The company’s Q3 2022-2023 earnings call reported that sales declined drastically due to VBP. Medtronic’s most impacted business lines included the surgical division, cardiac ablation solutions, and neurovascular lines. According to estimates, VBP affected 80% of Medtronic’s product portfolio in China. As a response, the company reduced its marketing, selling, administrative costs, and discounts. However, by Q2 2023-2024, the company said it would work through the impact due to China VBP by the end of FY 2024.
VBP has also negatively affected other foreign medtech companies, such as Alcon in China. Alcon’s exposure to China is about 5% of the company’s sales, predominantly in the surgical division. Alcon is likely to witness the impact of VBP in FY2024, ending in March 2025. Since the rollout of VBP takes place on a province-by-province basis, an abrupt fall in sales is highly unlikely in Alcon’s implants segment.d
The introduction of VBP has unfavorably impacted domestic medtech companies manufacturing intraocular lenses (IOLs) in China. Local player Airui Technology decreased its price of IOLs by as much as 65%.
Pressure on resource availability
National-level VBP tenders in China are usually finalized and awarded quickly. For instance, a temporary alliance of 22 (out of 23) provinces issued a competitive tender for liver function tests (LFTs) in November 2022. This tender’s result was published on December 30, 2022.
Compared to biopharma players, an idiosyncratic challenge for medtech companies is the need for a considerable amount of resources to be readily available when tenders occur. These resources must be strategically deployed to facilitate responses to tenders that are issued on short notice and awarded quickly and frequently. This is particularly challenging when bidding for regional or provincial VBP tenders that occur more frequently than national tenders, as many provinces in the country award contracts at different times.
Market exits
Although in the minority, some companies opted for more extreme measures. A few of them decided to withdraw from the Chinese market. For example, in March 2023, US-based Zimvie disclosed its intention to withdraw its spine business from the Chinese market following the challenges caused by the VBP roll-out.
Medtech companies have adapted amidst tough market conditions
Medtech companies faced pressure to lower prices, adjust their market and segment entry strategies, and optimize their workforce. These strategic changes aimed at mitigating the impact of lower revenue and profit margins on their existing product lines.
Staffing and organizational changes
Many medical device manufacturers that have encountered significant price reductions have responded by either reducing their workforce to manage expenses or by maintaining only managerial roles to focus on distribution in markets outside of VBP. Similarly, some medtech companies that witnessed moderate price cuts streamlined their field force to better align with the future servicing needs of their customers, including hospitals.
Other staffing and organizational changes include freezing hiring, outsourcing technical service jobs to third-party providers, and consolidating multiple product-focused teams into a single team.
Business model alterations
Some MNCs, including in-vitro diagnostics (IVD) companies, have changed their business model and leveraged partnerships with local medtech players to develop products. This strategic move will likely attenuate margin pressure by utilizing the local medtech partner’s cost advantage. Another advantage is that in-licenses or combined medical device development will likely counteract revenue stream losses.
IVD players are increasingly partnering with local medtech players to minimize the risk to their business model due to VBP, increase profit margins, expand their revenue streams, and continue to have sustainable relationships with hospitals. For example, Roche Diagnostics partnered with Fapon Biotech in November 2022 to improve its cost advantage by outsourcing its non-core reagent materials. Similarly, Danaher partnered with China Resources to outsource portfolios to national distributors or contract sales organizations in 2022.
VBP brought a mixed bag of consequences to other medtech industry stakeholders
Impact on the Chinese government spending
The Chinese government made considerable savings through the VBP program, thanks to curbing certain healthcare costs, and could potentially shift these savings to the sector’s other segments. The estimated annual savings based on the intended purchase volume was 10.9 billion yuan (US$1.6 billion) for coronary stents, 16 billion yuan (US$2.3 billion) for artificial hip and knee joints, and 26 billion yuan (USD$3.7 billion) for spinal and orthopedic products.
To put these numbers into perspective, the total savings from these three categories alone make up nearly 2.2% of China’s total public medical insurance spending of US$372.6 billion in 2021. These savings stem from VBP’s aim to reduce healthcare expenditures for the Chinese government by providing reasonably priced medical devices and implementing standardized pricing nationwide.
Impact on medtech distributors
One of the primary reasons for the high costs of medical devices for hospitals and patients in China is the unreasonably elevated profit margins of medtech distributors. The introduction of VBP has negatively impacted these margins.
At the same time, medtech companies are likely to pivot from a distributor-driven model to a direct distribution strategy to regain their own margins lost due to the increasing price pressures imposed by VBP. This transition is likely to limit the role of distributors to logistics functions, as seen in many other markets. At the same time, medtech companies will take ownership of commercial responsibilities and execute them through various channels.
The focus of VBP has expanded to specialized medical device categories
While the initial focus of VBP was on commoditized products with strong local alternatives, VBP has now ventured into medical device categories earlier perceived as not feasible for VBP tenders, such as electrophysiology for cardiology and immunoassays for IVD.
With VBP causing a radical change in commoditized products, medtech companies must now speed up registration and commercialization of products from specialized (non-commoditized) medical device categories that are in the pipeline. Pharmaceutical companies have already embraced this shift in strategy, while the change is gradually gaining steam among medtech players.
EOS Perspective
VBP is not a win-win strategy for all medtech stakeholders. Clear winners of VBP are patients and the Chinese healthcare system, while medtech companies, both domestic and foreign, and medtech distributors might get the shorter end of the stick.
VBP has made it difficult for all medtech companies operating in China to earn profits as high as in the past and has forced them to navigate in a more challenging environment.
At the same time, VBP is not entirely synonymous with foreign medtech players not succeeding in China. Chinese patients tend to prefer medical devices made by Western producers over those from domestic companies, provided that the imported devices are available at affordable prices. This preference is mainly due to the perception that foreign products are of higher quality than local options.
VBP will likely foster innovation in technology as companies will need to develop and design the best quality products to have an edge over their competition in tenders. Due to lowered prices, the bargaining power of medtech companies has decreased. Therefore, to differentiate themselves from their competition, they will need to prioritize innovation.
Although VBP has increased headwinds on the prices of medical devices, it has fueled strategic partnerships of MNCs with local medtech players. Local partnerships are likely a good move for all involved stakeholders, potentially also driving the overall growth of the medtech industry in China.
With China’s intention to pay 80% of its medtech expenditure through VBP by 2025, it will not be surprising to see VBP’s rollout in new categories of medical devices in the country.
The introduction of VBP will also have global repercussions, including a decline in small to medium medtech players’ interest in entering the Chinese market. However, the undeniable advantage of VBP’s introduction is that medtech companies will strive to innovate at lower costs, which will be a long-term driver for the market.